Cannabinol (CBN) is one of the five more common cannabinoids found in cannabis. This active substance is widely present in the plant as acid form (CBNA) and, upon heating it is converted to the final product. CBN represents the non-enzymatic (spontaneous) reaction of THC oxidation and is often defined as an “artifact” of THC itself, derived from a reaction known as aromatization occurring spontaneously in cannabis extracts, probably as a result of prolonged or poor-quality storage conditions. CBN is present in cannabis’ dried flowers only in trace amounts (around 1% vs 30% of THC) and shows about 1⁄4 of THC’s potency.
Medical
16/05/2019
What is CBN and what does it do in cannabis?
CBN was the first cannabinoid to be discovered
CBN was the first cannabinoid isolated from cannabis: the history of CBN dates back to 1896, when this compound was identified and isolated for the first time from the exuded resin of Indian hemp defined with the term “charas”. Since then, scientists have further examined cannabinol, and have found that it comprises a mixture of compounds with similar physical characters, which are at present day, included in the subfamily of “CBN-type compounds”.
Fig.1
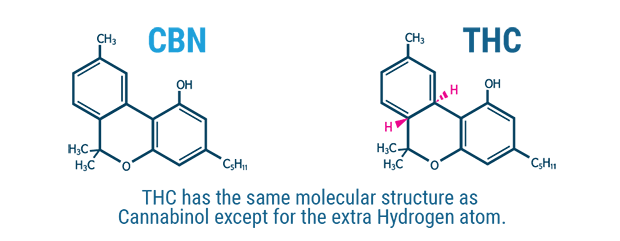
The structural similarities between CBN and THC
Its structure, chemically similar to that of Δ9-THC, was reported for the first time in 1940, and for two decades CBN was the only cannabinoid to be structurally explained.
Compared to Δ9-THC, the presence of an aromatic ring confers to CBN molecule a very high stability. Due to this characteristic, CBN has been used as a marker for the identification of narcotic cannabis in archeological findings.
CBN shows a weak psychoactive power, and similarly to THC it binds to cannabinoids receptors (CB1-R and CB2-R), with a higher affinity for CB2-R, mainly expressed on a variety of immune cells, such as lymphocytes (T and B), macrophages and dendritic cells.
The medicinal properties of CBN
From the medical point of view, CBN has shown a variety of properties:
– Antibiotic and antibacterial – especially against a variety of methicillin-resistant Staphylococcus aureus (MRSA) strains.
– Immunosuppressive and anti-inflammatory – by stimulating CB2-R thus triggering the programmed death (apoptosis) in immune cells and inhibiting the production of a variety of cytokines (released by cells especially of the immune system and involved, among others in inflammatory processes).
– Appetite stimulant – probably by interacting, with a weak affinity (about 10%) with both cannabinoid receptors (CB1 and CB2) of the human endocannabinoid system. Such an effect is further potentiated by Δ9THC, that, when co-administered, may synergistically induce powerful hyperphagic and anti-anorexic effects.
– Ocular tension lowering – especially after chronic administration
– Topical skin agent – CBN has potential as a component in topical applications, inhibiting keratinocyte proliferation via CB-R independent mechanisms, suggesting a potential role in psoriasis.
– Anticancer – (breast and ovarian cancer in particular) and bone formation – by stimulating the recruitment of marrow mesenchymal stem cells in a quiescent (inactive) state.
– Sleeping aid – such an activity is mainly attributed to CBN derivatives (rather than to CBN itself) exhibiting a significant prolongation of sleeping time and catalepsy. In a 1975 study on the effects of interaction between D9-THC and CBN, 5 male volunteers have referred an enhancement of drowsy feelings, among others, when CBN was administered in combination with D9-THC, compared to THC alone (of note, such effects were not observed when volunteers have received CBN alone).
Bibliography:
- Appendino G, Gibbons S, Giana A, et al. J Nat Prod. 2008;71(8):1427-30.
- Colasanti BK, et al. Exp Eye Res. 1984;39(3):251-9.
- Croxford JL, Yamamura T. J Neuroimmunol. 2005;166(1-2):3-18.
- Da Cheng H, et al. Medicinal Plants: Chemistry Biology and Omics; 2015:217-251.
- El-Alfy A, et al. Pharmacol Biochem Behav. 2010 ; 95(4): 434–442
- Farrimond JA, Whalley BJ, Williams CM. Psychopharmacology (Berl). 2012;223(1):117-29.
- Hanuš LO, Meyer SM, Muñoz E, et al. Nat Prod Rep. 2016;33(12):1357-1392.
- Karniol IG, et al. Pharmacology. 1975; 13:502-512
- Morales P, et al. Prog Chem Org Nat Prod. 2017;103: 103-131
- Russo EB, et al. Adv Pharmacol. 2017;80:67-134.
- Sharafi G, et al. Journal of Pancreatic Cancer 2019; 5.1
- Wood TB et al. III – Cannabinol. Part I. Journal of the Chemical Society, Transactions.1899; 75: 20-36.
- Yoshida H, et al. Chem Pharm Bull (Tokyo). 1995;43(2)-335-7.
- Zygmunt PM, Andersson DA and Högestätt ED. J Neurosci 2002, 22 (11):4720-4727
More articles you would like
Recommended
Tip us!
Do you have a tip or did you experience something amazing that you want to share with us? Send our team a message!
Your order is converted to euro's, however you will be able to select your desired currency in the payment process
Close